Dr. Andres de la Escosura belongs to the Organic Chemistry Department and the recently created Institute for Advanced Research in Chemical Sciences (IAdChem) at Universidad Autónoma de Madrid (UAM).
Our research group is concerned with the design and synthesis of new supramolecular and nanostructured hybrids from synthetic and biological components, and the study of their complex biological functions.
We are currently working in areas that include catalysis, reaction networks, replicative systems and prebiotic chemistry.
Sonia Vela-Gallego*, Zulay Pardo-Botero, Cristian Moya and Andres de la Escosura*: Chem. Sci., 2022, 13, 10715-10724
A major challenge for understanding the origins of life is to explore how replication networks can engage in an evolutionary process.
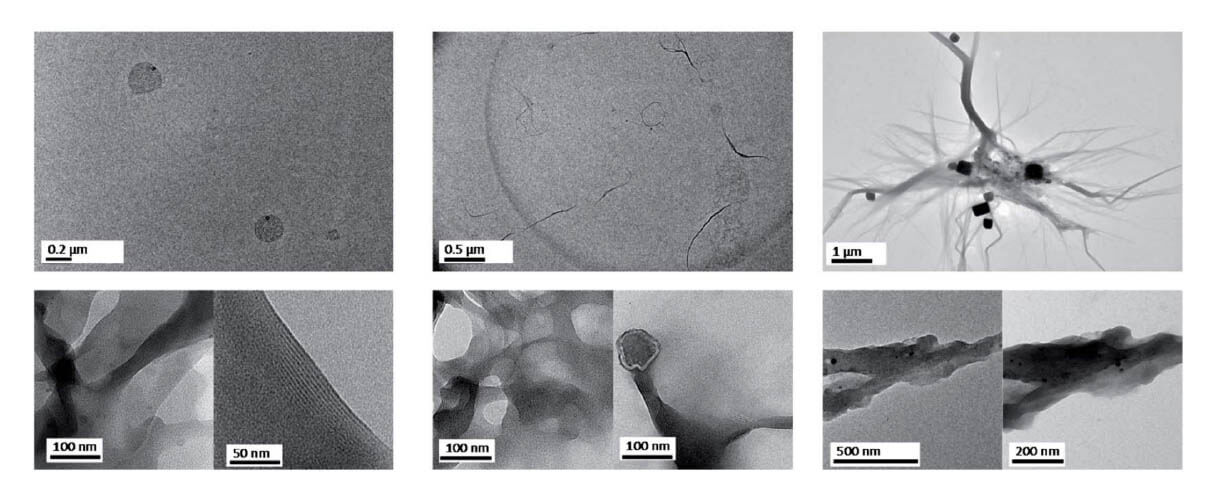
Herein, we shed light on this problem by implementing a network constituted by two different types of extremely simple biological components: the amino acid cysteine and the canonical nucleobases adenine and thymine.
Verónica Almeida-Marrero, Fleur Bethlehem, Sara Longo, M. Candelaria Bertolino, Tomás Torres*, Jurriaan Huskens*, Andrés de la Escosura*: Angew. Chem. Int. Ed. 2022, 134, e202206900
The modification of surfaces with multiple ligands allows the formation of platforms for the study of multivalency in diverse processes.
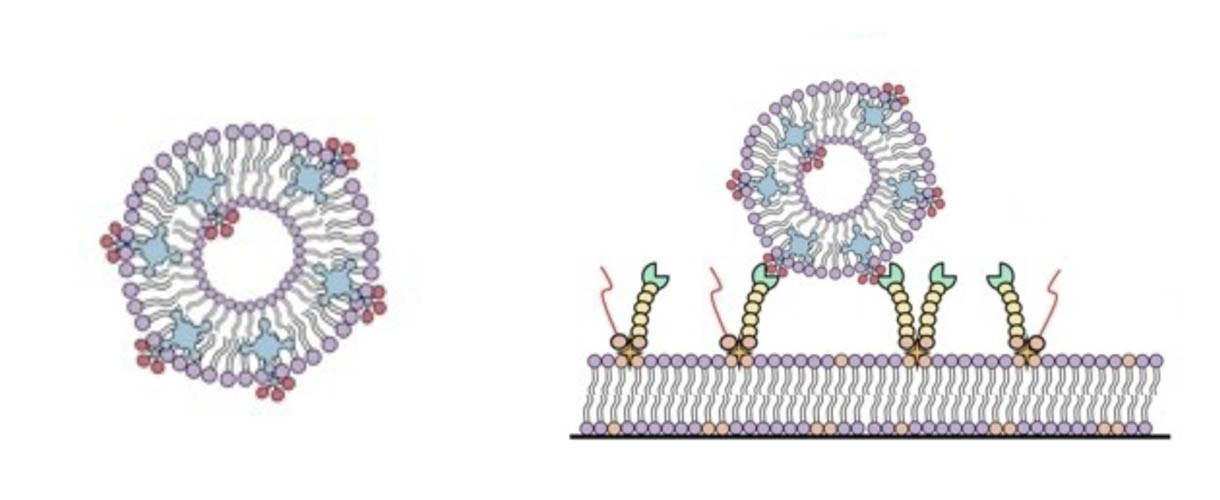
Herein we use this approach for the implementation of a photosensitizer (PS)-nanocarrier system that binds efficiently to siglec-10, a member of the CD33 family of siglecs (sialic acid (SA)-binding immunoglobulin-like lectins).
The biohybrid systems with human siglec-10-displaying supported lipid bilayers (SLBs) therefore acts as an excellent cell membrane mimic, while the binding with PS-loaded SUVs shows the potential for targeting siglec-expressing cells with photosensitizing nanocarriers.
A. Kumar-Bandela, N. Wagner, S. Morales-Reina, H. Sadihov, A. Chotera-Ouda, K. Basu, R. Cohen-Luria, A. de la Escosura*, G. Ashkenasy: Proc. Natl. Acad. Sci. USA 2021, 118, e2015285118
Research on the chemical origin of life comprises one of the most exciting topics in contemporary science. Prebiotic chemistry provided evidence that precursors of both nucleic acids and proteins might be formed in the prebiotic environment.
We show here that short nucleopeptide chimeras can replicate through autocatalytic and cross-catalytic processes, governed synergistically by the hybridization of the nucleobase motifs and the assembly propensity of the peptide segments.
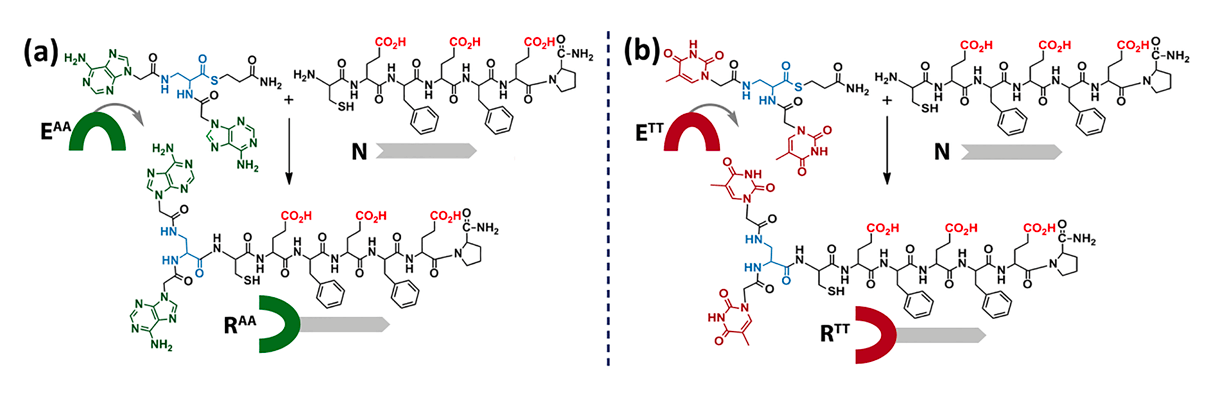
Unequal assembly-dependent replication induces clear selectivity toward the formation of a certain species within small networks of complementary nucleopeptides.
The selectivity pattern may be influenced and indeed maximized to the point of almost extinction of the weakest replicator when the system is studied far from equilibrium and manipulated through changes in the physical (flow) and chemical (template and inhibition) conditions. We postulate that similar processes may have led to the emergence of the first functional nucleic-acid–peptide assemblies prior to the origin of life.
Andrés de la Escosura Research Group | 2023®